New approach methodologies in human regulatory toxicology – Not if, but how and when! - ScienceDirect
Por um escritor misterioso
Last updated 14 janeiro 2025


Distinguishing between expert and statistical systems for application under ICH M7 - ScienceDirect

Pharmaceutical toxicology: Designing studies to reduce animal use, while maximizing human translation - ScienceDirect

Integrative systems toxicology to predict human biological systems affected by exposure to environmental chemicals - ScienceDirect

Toxicogenomics: A 2020 Vision: Trends in Pharmacological Sciences

Transgenic Mouse Models Transferred into the Test Tube: New Perspectives for Developmental Toxicity Testing In Vitro?: Trends in Pharmacological Sciences
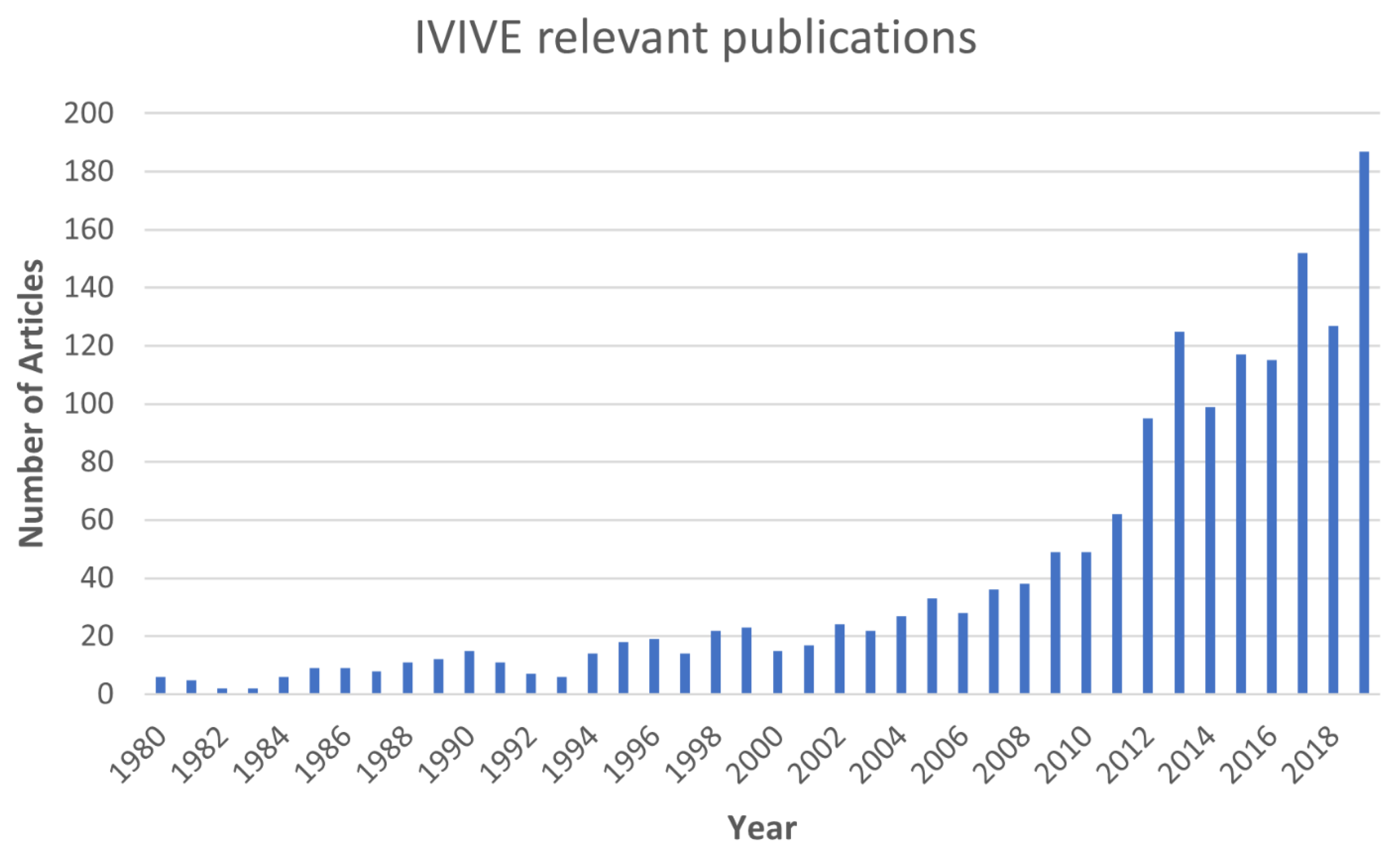
Toxics, Free Full-Text

Assessing Toxicity with Human Cell-Based In Vitro Methods: Trends in Molecular Medicine

New Approach Methodologies (NAMs) for safety testing of complex food matrices: A review of status, considerations, and regulatory adoption - ScienceDirect
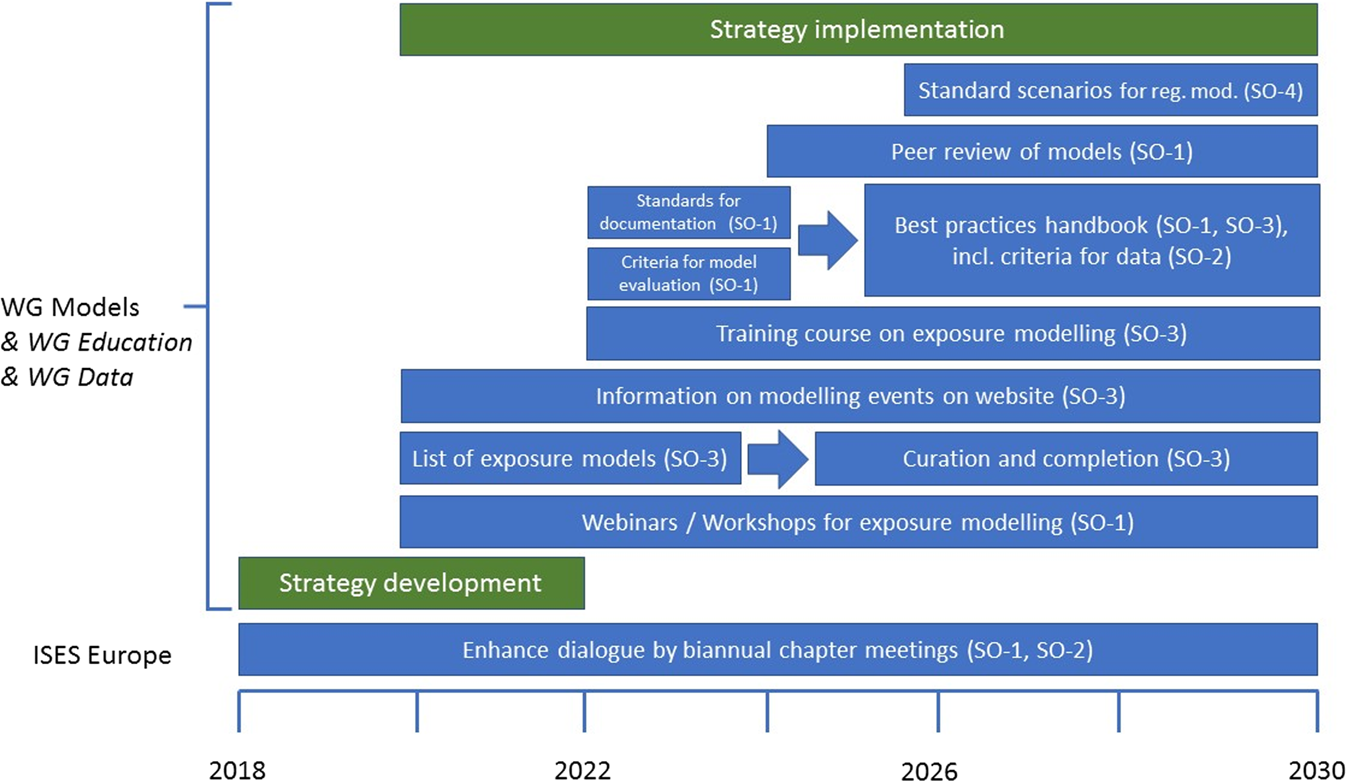
Exposure modelling in Europe: how to pave the road for the future as part of the European Exposure Science Strategy 2020–2030

In silico toxicology protocols - ScienceDirect

Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? - ScienceDirect

Regional empowerment through decentralised governance under a centralised regulatory system facilitates the development of cellular therapy in China - The Lancet Haematology

Japan-Specific Key Regulatory Aspects for Development of New Biopharmaceutical Drug Products - Journal of Pharmaceutical Sciences

Innovation in regulatory approaches for endocrine disrupting chemicals: The journey to risk assessment modernization in Canada - ScienceDirect
Recomendado para você
-
 Birth date between 2003-01-01 and 2003-12-31 (Sorted by Popularity Ascending)14 janeiro 2025
Birth date between 2003-01-01 and 2003-12-31 (Sorted by Popularity Ascending)14 janeiro 2025 -
 About: Mary Ellen Lepionka - Indigenous History of Essex County, Massachusetts14 janeiro 2025
About: Mary Ellen Lepionka - Indigenous History of Essex County, Massachusetts14 janeiro 2025 -
 Quem é a esposa de Regis Danese? Cantora gospel já apareceu na Globo14 janeiro 2025
Quem é a esposa de Regis Danese? Cantora gospel já apareceu na Globo14 janeiro 2025 -
 Kily González - Wikipedia14 janeiro 2025
Kily González - Wikipedia14 janeiro 2025 -
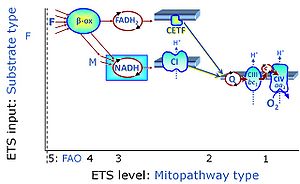 Fatty acid oxidation pathway control state - Bioblast14 janeiro 2025
Fatty acid oxidation pathway control state - Bioblast14 janeiro 2025 -
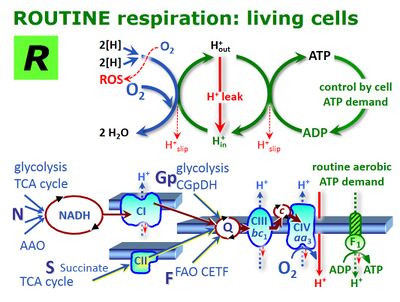 ROUTINE respiration - Bioblast14 janeiro 2025
ROUTINE respiration - Bioblast14 janeiro 2025 -
 DHS grads talk, motivate, eat … and celebrate, News14 janeiro 2025
DHS grads talk, motivate, eat … and celebrate, News14 janeiro 2025 -
 Augite: Mineral information, data and localities.14 janeiro 2025
Augite: Mineral information, data and localities.14 janeiro 2025 -
Development of Adverse Outcome Pathway for PPARγ Antagonism Leading to Pulmonary Fibrosis and Chemical Selection for Its Validation: ToxCast Database and a Deep Learning Artificial Neural Network Model-Based Approach14 janeiro 2025
-
 Kelly Godoy - Jornalista. Apresentadora e editora da Record News #108 - Fala Pô Podcast14 janeiro 2025
Kelly Godoy - Jornalista. Apresentadora e editora da Record News #108 - Fala Pô Podcast14 janeiro 2025
você pode gostar
-
 r/Diep2io14 janeiro 2025
r/Diep2io14 janeiro 2025 -
 DICAS COMO DAR ESPAÇO (SEPARAR) SEU NICK NO FREE- FIRE SAIBA COMO RAPIDO E FACIL!!14 janeiro 2025
DICAS COMO DAR ESPAÇO (SEPARAR) SEU NICK NO FREE- FIRE SAIBA COMO RAPIDO E FACIL!!14 janeiro 2025 -
 Indiana Jones 5 reviews: Reactions, Rotten Tomatoes score & more14 janeiro 2025
Indiana Jones 5 reviews: Reactions, Rotten Tomatoes score & more14 janeiro 2025 -
 🌱🥛 Leche on X: shiny mew and mewtwo gettin along #pokemon #mewtwo #mew / X14 janeiro 2025
🌱🥛 Leche on X: shiny mew and mewtwo gettin along #pokemon #mewtwo #mew / X14 janeiro 2025 -
 Super hero comic starblast explosion icon dialogue cloud aesthetic png download Stock Illustration14 janeiro 2025
Super hero comic starblast explosion icon dialogue cloud aesthetic png download Stock Illustration14 janeiro 2025 -
 Como dizer tudo em inglês: livro de atividades14 janeiro 2025
Como dizer tudo em inglês: livro de atividades14 janeiro 2025 -
 Stream Enjoy the Ultimate Football Experience with FIFA 20 APK +14 janeiro 2025
Stream Enjoy the Ultimate Football Experience with FIFA 20 APK +14 janeiro 2025 -
 TOP 50 MELHORES NICKS QUE DÃO MEDO PRA COLOCAR NO FF SÓ NICK INSANO!!!😳😳14 janeiro 2025
TOP 50 MELHORES NICKS QUE DÃO MEDO PRA COLOCAR NO FF SÓ NICK INSANO!!!😳😳14 janeiro 2025 -
 Tate No Yuusha No Nariagari by Cholil Iruka14 janeiro 2025
Tate No Yuusha No Nariagari by Cholil Iruka14 janeiro 2025 -
 80's & 90's Dance, Techno & Disco Music Hits : r/spotify14 janeiro 2025
80's & 90's Dance, Techno & Disco Music Hits : r/spotify14 janeiro 2025
