8 (4) A, C 0.1 gm of organic compound was analysed by Kjeldahl's method. In analysis produced NH, absorbed in 30 ml N/5 H,SO. The remaining acid required 20 ml N/10 NaOH
Por um escritor misterioso
Last updated 31 março 2025

Click here:point_up_2:to get an answer to your question :writing_hand:84 a c01 gm of organic compound was analysed bykjeldahls method in analysis produced nhabsorbed

Chemical Engg Reviewer, PDF, Hydroxide
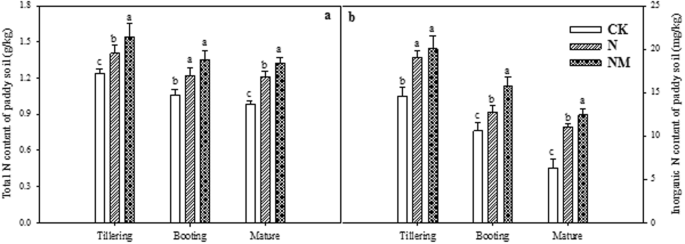
The role of Chinese Milk Vetch as cover crop in complex soil nitrogen dynamics in rice rotation system of South China
53 Ammonia obtained from 0.4 g of an organic compound by kjeldahl's method was absorbed in 30 ml of 0.25 M H2So4 . The excess acid was neutralized by 30 ml of

REVIEW OF FEATURES OF MERCURY CHEMISTRY OF CHIEF INTEREST TO RADIOCHEMISTS, Radiochemistry of Mercury
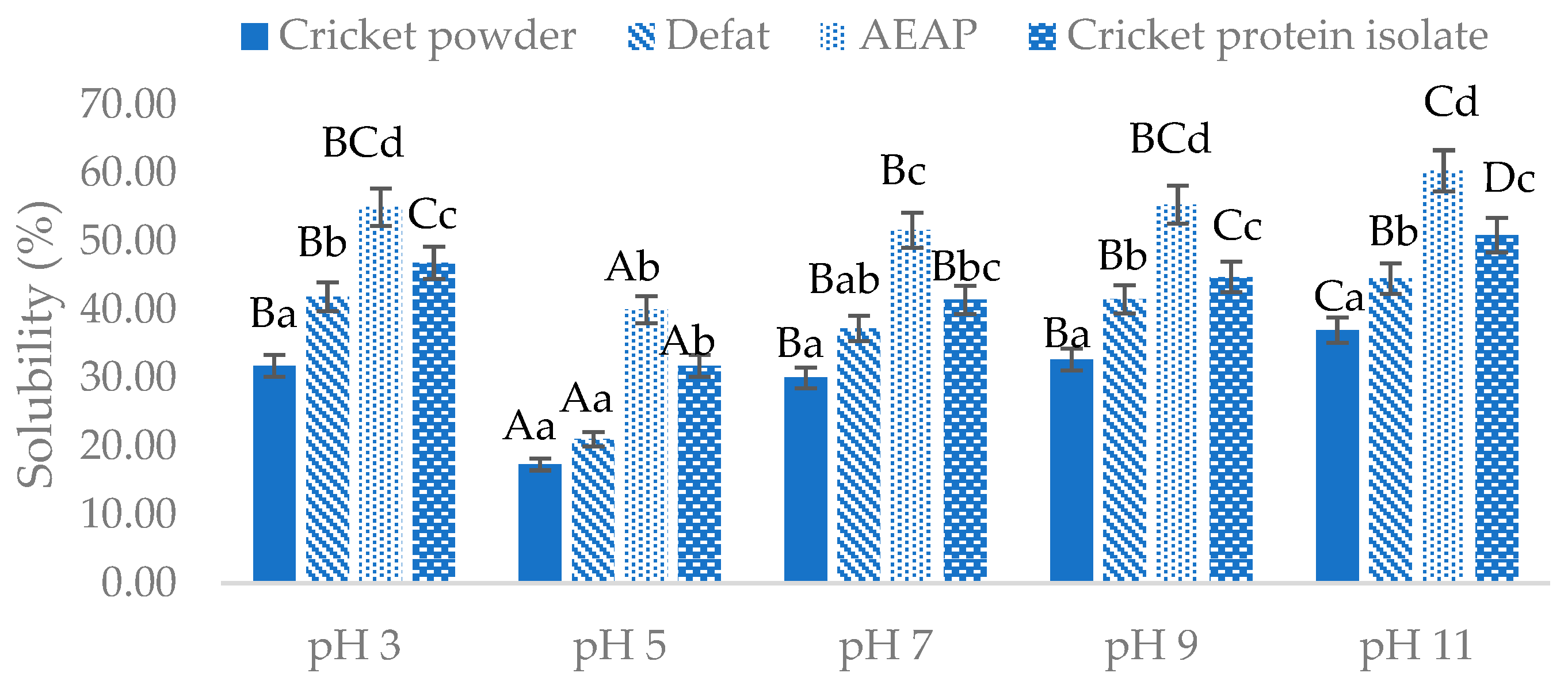
Foods, Free Full-Text

The Titration in the Kjeldahl Method of Nitrogen Determination: Base or Acid as Titrant?

1 0.1 g of organic compound in Kjeldahl method released ammonia gas which exactly neutralised 2 mL of 0.5 M sulphuric acid. Find percentage of nitrogen. (28 %)

0.35 g of an organic substance was Kjeldahlised and the ammonia obtained was passed into 100ml of M/10H_2SO_4 the excess acid required 154ml of M/10NaOH neutralisation calculate the % of nitrogen in

NR. Ammonia obtained from 0.4 g of an organic substance by Kjeldahl's method was absorbed in 30 ml. of 0-25 MH SO. The excess of the acid was neutralized by the addition

Doc 117 b p s xi chemistry iit jee advanced study package 2014 15 by S.Dharmaraj - Issuu
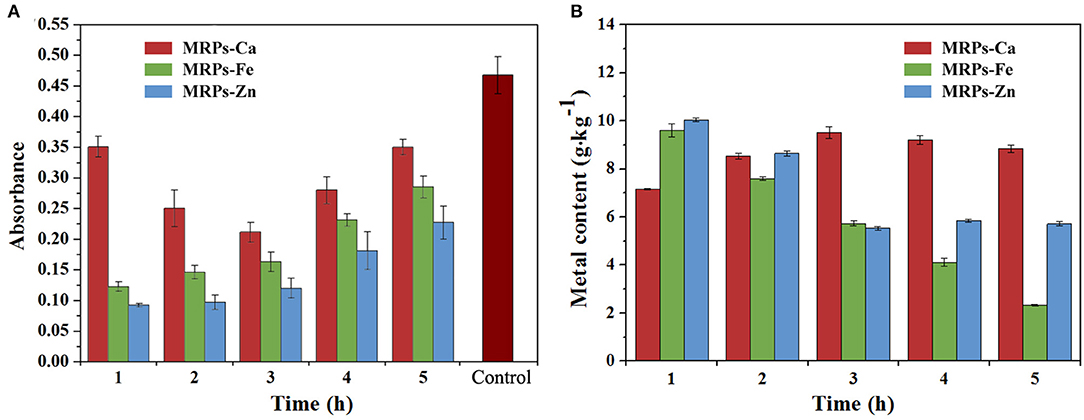
Frontiers Nano-Sized Antioxidative Trimetallic Complex Based on Maillard Reaction Improves the Mineral Nutrients of Apple (Malus domestica Borkh.)
Recomendado para você
-
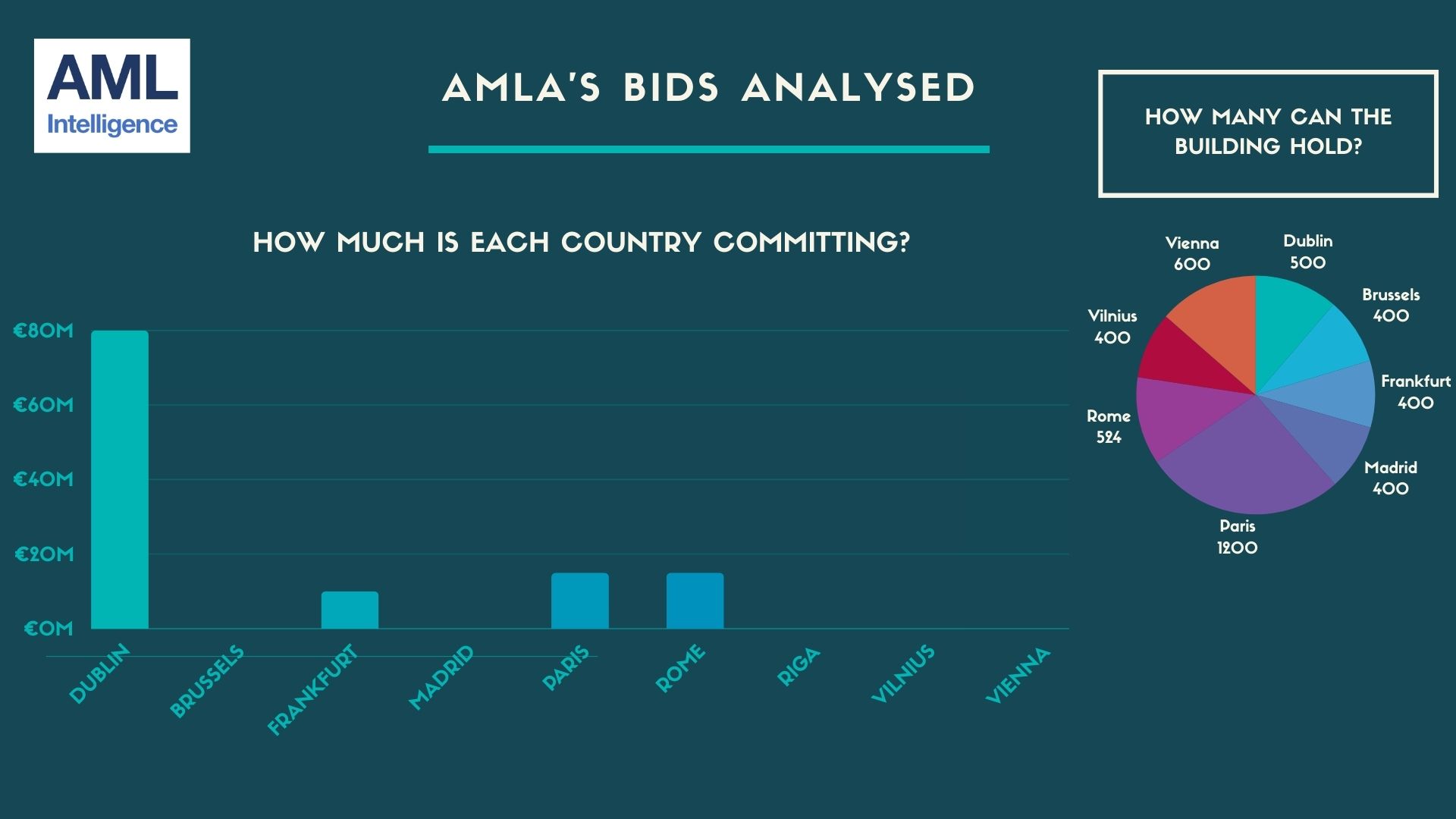 INSIGHT: Each of the nine AMLA bids analysed – we crunch the numbers and get behind the details as race hots up - AML Intelligence31 março 2025
INSIGHT: Each of the nine AMLA bids analysed – we crunch the numbers and get behind the details as race hots up - AML Intelligence31 março 2025 -
 b) A sample of tin is analysed in a time of flight mass spectrometer. The sample is ionised by electron31 março 2025
b) A sample of tin is analysed in a time of flight mass spectrometer. The sample is ionised by electron31 março 2025 -
 Retention time of phenolic compounds standards analysed by HPLC31 março 2025
Retention time of phenolic compounds standards analysed by HPLC31 março 2025 -
The stakes of inclusive education, analysed by Liesbeth Roolvink from Sightsavers31 março 2025
-
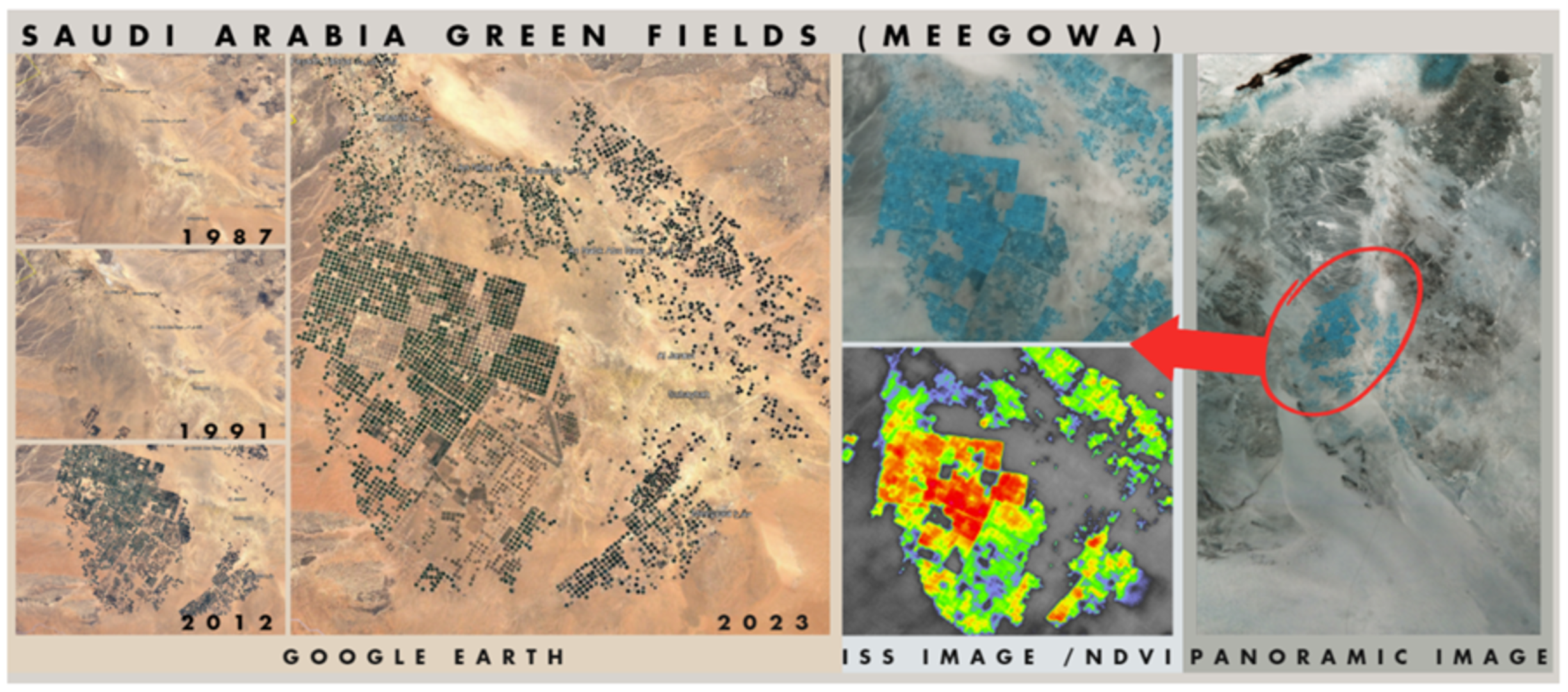 ESA - Saudi Arabia Green Fields, image analysed by team Aretusa31 março 2025
ESA - Saudi Arabia Green Fields, image analysed by team Aretusa31 março 2025 -
 Number of units of analysis selected and analysed in the primary31 março 2025
Number of units of analysis selected and analysed in the primary31 março 2025 -
 India Analysed: Buy India Analysed by Kakar Sudhir at Low Price in India31 março 2025
India Analysed: Buy India Analysed by Kakar Sudhir at Low Price in India31 março 2025 -
 ID systems analysed: e-Estonia31 março 2025
ID systems analysed: e-Estonia31 março 2025 -
Visualiser for analysed entities · microsoft presidio · Discussion #1026 · GitHub31 março 2025
-
 Sergio Reguilón's stop-start Manchester United career analysed31 março 2025
Sergio Reguilón's stop-start Manchester United career analysed31 março 2025
você pode gostar
-
 VSCO Girl Uno Hearts Sticker - Sticker Mania31 março 2025
VSCO Girl Uno Hearts Sticker - Sticker Mania31 março 2025 -
 Autocolante encorajador escrito à mão na tradução para o português do brasil nunca, nunca, nunca desista31 março 2025
Autocolante encorajador escrito à mão na tradução para o português do brasil nunca, nunca, nunca desista31 março 2025 -
 Tất cả truyện - Thiên hà truyện đa dạng thể loại - cập nhập nhanh nhất31 março 2025
Tất cả truyện - Thiên hà truyện đa dạng thể loại - cập nhập nhanh nhất31 março 2025 -
 Comentários, Hangman por - 14 de Março de 201531 março 2025
Comentários, Hangman por - 14 de Março de 201531 março 2025 -
 Mime and Dash by TheSketcherD on DeviantArt31 março 2025
Mime and Dash by TheSketcherD on DeviantArt31 março 2025 -
 AS Roma And Slavia Prague Win Big In Group G Of Europa League31 março 2025
AS Roma And Slavia Prague Win Big In Group G Of Europa League31 março 2025 -
 Ssd 1tb Up Gamer : Será que Presta? vamos ver nesse vídeo.31 março 2025
Ssd 1tb Up Gamer : Será que Presta? vamos ver nesse vídeo.31 março 2025 -
 Godzillaearth 3D models - Sketchfab31 março 2025
Godzillaearth 3D models - Sketchfab31 março 2025 -
 SCP-582 Villains+BreezeWiki31 março 2025
SCP-582 Villains+BreezeWiki31 março 2025 -
 Design your roblox avatar by Abigail03631 março 2025
Design your roblox avatar by Abigail03631 março 2025
