Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Last updated 11 abril 2025

Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.

Lab Information

Biotech Fierce Biotech

Real world single center experience on the efficacy of stopping
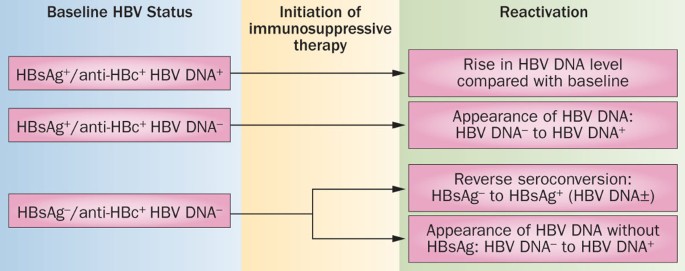
Management of patients with hepatitis B who require

Landon Loving on LinkedIn: Selecta, Sobi rout gout in pair of
LA Weekend: Haunted Hayride; Jack-O'-Lantern Walk; Pull-A-Plane

HBV replication inhibitors. - Abstract - Europe PMC

IHEP (International Hepatology Education Program)

Landon Loving on LinkedIn: Selecta, Sobi rout gout in pair of

Journal of Medicinal Chemistry

33rd Annual Meeting & Pre-Conference Programs of the Society for

HBV replication inhibitors. - Abstract - Europe PMC

Can Medivation's CEO get a bidding war started?

Antios Therapeutics' ATI-2173 Demonstrates Suppression of
Recomendado para você
-
Steam Workshop::Roblox - Doors NEXTBOTS (SEEK UPDATE)11 abril 2025
-
 Halt from Roblox Doors by KwamisLovesHearts on DeviantArt11 abril 2025
Halt from Roblox Doors by KwamisLovesHearts on DeviantArt11 abril 2025 -
Workshop di Steam::[DrGBase] Roblox DOORS Nextbots11 abril 2025
-
 Halt From Doors11 abril 2025
Halt From Doors11 abril 2025 -
 The E-Scooters Loved by Silicon Valley Roll Into New York11 abril 2025
The E-Scooters Loved by Silicon Valley Roll Into New York11 abril 2025 -
 Tanglin Halt Food Centre To Close On 31 Jul, Visit Margaret Drive Hawker Centre Instead11 abril 2025
Tanglin Halt Food Centre To Close On 31 Jul, Visit Margaret Drive Hawker Centre Instead11 abril 2025 -
Steam Workshop::Avalash11 abril 2025
-
 Adweek's Most Powerful Women in Sports 202011 abril 2025
Adweek's Most Powerful Women in Sports 202011 abril 2025 -
 Road Test Review - 2015 Hyundai Genesis 5.011 abril 2025
Road Test Review - 2015 Hyundai Genesis 5.011 abril 2025 -
 A not so bright Christmas? High electric rates take 'heartbreaking11 abril 2025
A not so bright Christmas? High electric rates take 'heartbreaking11 abril 2025
você pode gostar
-
 Paper.io — The Best Multiplayer Game By Voodoo11 abril 2025
Paper.io — The Best Multiplayer Game By Voodoo11 abril 2025 -
 A Plague Tale: Requiem Collectible Guide (flowers, feathers11 abril 2025
A Plague Tale: Requiem Collectible Guide (flowers, feathers11 abril 2025 -
 Haikyuu tem 4ª temporada anunciada! - IntoxiAnime11 abril 2025
Haikyuu tem 4ª temporada anunciada! - IntoxiAnime11 abril 2025 -
 Fred Benson, Wikia Stranger Things11 abril 2025
Fred Benson, Wikia Stranger Things11 abril 2025 -
 Ocarina of Time Ganandorf and Link (and is Ocarina of time, Link costume, Link zelda costume11 abril 2025
Ocarina of Time Ganandorf and Link (and is Ocarina of time, Link costume, Link zelda costume11 abril 2025 -
 LGBT+ Flag Quiz Pop'n'Olly11 abril 2025
LGBT+ Flag Quiz Pop'n'Olly11 abril 2025 -
 HIGHSCHOOL OF THE DEAD episode ten and eleven: HOTD meets HOTD11 abril 2025
HIGHSCHOOL OF THE DEAD episode ten and eleven: HOTD meets HOTD11 abril 2025 -
 Isekai Nonbiri Nouka - Episódios - Saikô Animes11 abril 2025
Isekai Nonbiri Nouka - Episódios - Saikô Animes11 abril 2025 -
 Vegeta SSJ4(Baby Arc) vs Vegito SSJ3(Z) vs Goku SSJG vs Gogeta11 abril 2025
Vegeta SSJ4(Baby Arc) vs Vegito SSJ3(Z) vs Goku SSJG vs Gogeta11 abril 2025 -
onde assistir kimetsu no yaiba 3 dublado no hinata soul|Pesquisa11 abril 2025

![Workshop di Steam::[DrGBase] Roblox DOORS Nextbots](https://steamuserimages-a.akamaihd.net/ugc/1817787440847520331/BFF9386320C97DEBDD4EFE6FA748903CB5C29C6C/?imw=637&imh=358&ima=fit&impolicy=Letterbox&imcolor=%23000000&letterbox=true)

