What You Should Know About CSV in Pharma
Por um escritor misterioso
Last updated 07 abril 2025

Learn more about computer system validation, which is required by the FDA and other global regulatory bodies for drug and medical device manufacturers.
Download Program, Are you compliant with FDA requirements for CSV, Data Integrity & Process Validation? Today's regulators are applying more

CSV, Data Integrity & Process Validation Virtual Summit, 2021

How Life Sciences Organizations can stand the test of compliance with Computer System Validation

Data Integrity in the Pharmaceutical Industry

What is Computer System Validation?

A Guide to CSV & CSA - Why The Shift?
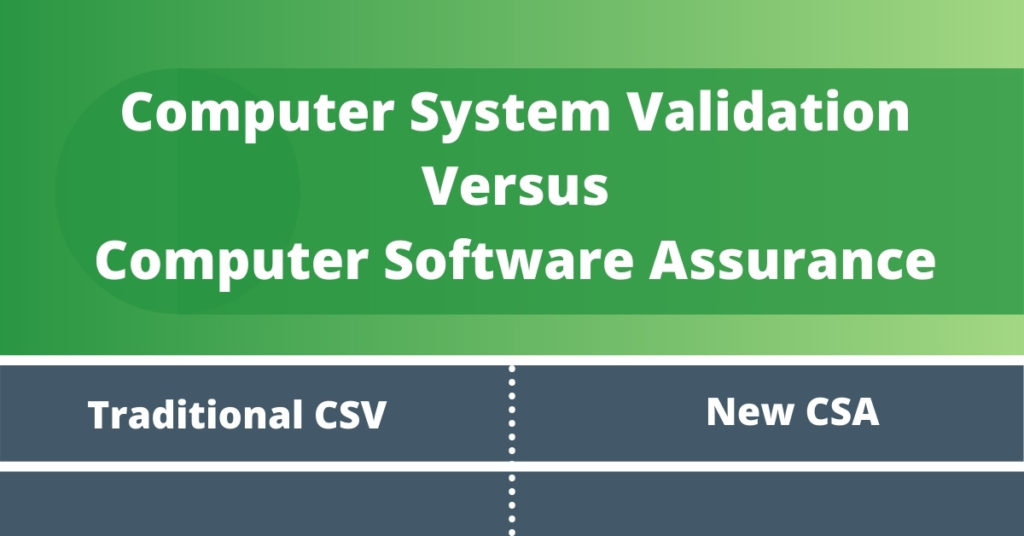
Infographic: Computer System Validation Vs. Computer Software Assurance - SL Controls

SAP S/4HANA Implementation and Validation - Life-Sciences-Alliance
Preparing for the GAMP Transition to Computer Software Assur

CSV - Computer System Validation

CSV vs. CSA: What Are the Main Differences?

How To Complete Computer Systems Validation (FDA)

Computer System Validation
Recomendado para você
-
CSVP - MARANhHÃO07 abril 2025
-
 DataMap PO SmartView Coupa App Marketplace07 abril 2025
DataMap PO SmartView Coupa App Marketplace07 abril 2025 -
 CRVPM Level VI/Certified AICPA SOC® Report Analyst (CASRA07 abril 2025
CRVPM Level VI/Certified AICPA SOC® Report Analyst (CASRA07 abril 2025 -
 CSV1A CYCLE STOP VALVE – Cycle Stop Valves, Inc07 abril 2025
CSV1A CYCLE STOP VALVE – Cycle Stop Valves, Inc07 abril 2025 -
 Afiliados - SINEPE/MA07 abril 2025
Afiliados - SINEPE/MA07 abril 2025 -
 CSV File Format07 abril 2025
CSV File Format07 abril 2025 -
 Manipulating CSV data using Python. (Part 1)07 abril 2025
Manipulating CSV data using Python. (Part 1)07 abril 2025 -
 How to map CSV files - ProperSoft Inc. Knowledge Base07 abril 2025
How to map CSV files - ProperSoft Inc. Knowledge Base07 abril 2025 -
 CSV Upload07 abril 2025
CSV Upload07 abril 2025 -
 Computer System Validation (CSV) to Computer Software Assurance07 abril 2025
Computer System Validation (CSV) to Computer Software Assurance07 abril 2025
você pode gostar
-
1019010_bicicleta-houston-aro-26 -passeio-bristol-lance-21-marchas-com-cesta-br26lcl_m2_63665696661730784607 abril 2025
-
Pokemon Legends - Arceus - 28th Jan 2022 *Official Content Only07 abril 2025
-
blogspotcarreirasestradareal.com: Carreta da alegria já chegou em CON.LAFAIETE todo os finais de ano 2 carretas levam alegria a garotada com músicas e palhaços.07 abril 2025
-
![Plants vs. Zombies 3 Beta [Android] Full Walkthrough Gameplay](https://i.ytimg.com/vi/N7zkbIiEhaY/maxresdefault.jpg) Plants vs. Zombies 3 Beta [Android] Full Walkthrough Gameplay07 abril 2025
Plants vs. Zombies 3 Beta [Android] Full Walkthrough Gameplay07 abril 2025 -
 Shop Adopt Me Pets Roblox online07 abril 2025
Shop Adopt Me Pets Roblox online07 abril 2025 -
 DR.LIVESEY WALK BUT IT'S CHAINSAWMAN - Coub - The Biggest Video07 abril 2025
DR.LIVESEY WALK BUT IT'S CHAINSAWMAN - Coub - The Biggest Video07 abril 2025 -
Crazy Athletics - Summer Sports and Games - Official game in the Microsoft Store07 abril 2025
-
 ꒰ ꒰ 𔘓 5 free soft oc's for girl — gacha club07 abril 2025
꒰ ꒰ 𔘓 5 free soft oc's for girl — gacha club07 abril 2025 -
 Forbidden Planet Retro Vintage Classic Sci Fi Movie Poster 1956 Black and White Poster by Carol Japp - Fine Art America07 abril 2025
Forbidden Planet Retro Vintage Classic Sci Fi Movie Poster 1956 Black and White Poster by Carol Japp - Fine Art America07 abril 2025 -
 static.wikia.nocookie.net/roblox/images/a/a7/Scree07 abril 2025
static.wikia.nocookie.net/roblox/images/a/a7/Scree07 abril 2025


